Cathodic protection technology is a type of electrochemical protection technology, which applies an external current to the surface of the corroded metal structure. The protected structure becomes the cathode, thereby suppressing the electron migration that occurs during metal corrosion and avoiding or reducing the occurrence of corrosion.
Cathodic protection technology can be divided into sacrificial anode cathodic protection and impressed current cathodic protection. This technology is basically mature and widely used for corrosion control of metal structures such as steel pipelines, water pumps, cables, ports, ships, tank bottoms, coolers, etc. in soil, seawater, freshwater, and chemical media.
Sacrificial anode cathodic protection is the process of connecting two metals with different activities and placing them in the same electrolyte. The more active metal loses electrons and is corroded, while the less active metal receives electron protection. Due to the corrosion of highly active metals during this process, it is called sacrificial anode cathodic protection.
External current cathodic protection is achieved by changing the potential of the surrounding environment through an external power source, so that the potential of the equipment to be protected remains lower than that of the surrounding environment, thus becoming the cathode of the entire environment. In this way, the equipment to be protected will not corrode due to the loss of electrons.
Working principle
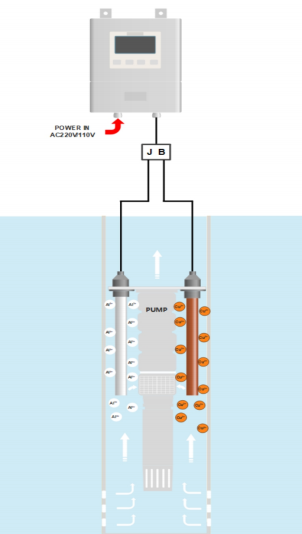
Use copper and aluminum alloys as anodes and the protected equipment system as cathodes. The copper ions obtained from electrolyzing copper anodes are toxic and form a toxic environment when mixed with seawater. The electrolytic aluminum anode produces Al3+, which forms Al (OH) 3 with the OH – produced by the cathode. This type of l (OH) 3 encapsulates the released copper ions and flows through the protected system with seawater. It has high adsorption capacity and can spread out into areas with slower seawater flow where marine organisms may reside, inhibiting their growth. When the copper aluminum anode system is electrolyzed in seawater, a dense layer of calcium and magnesium is formed on the inner surface of the steel pipeline as the cathode, and the aluminum hydroxide colloid generated by electrolysis flows with seawater, forming a protective film on the inner wall of the pipeline. The calcium magnesium coating and aluminum hydroxide colloidal film block the diffusion of oxygen, increase concentration polarization, and slow down the corrosion rate, which can achieve the purpose of anti fouling and anti-corrosion.
Post time: Aug-28-2025